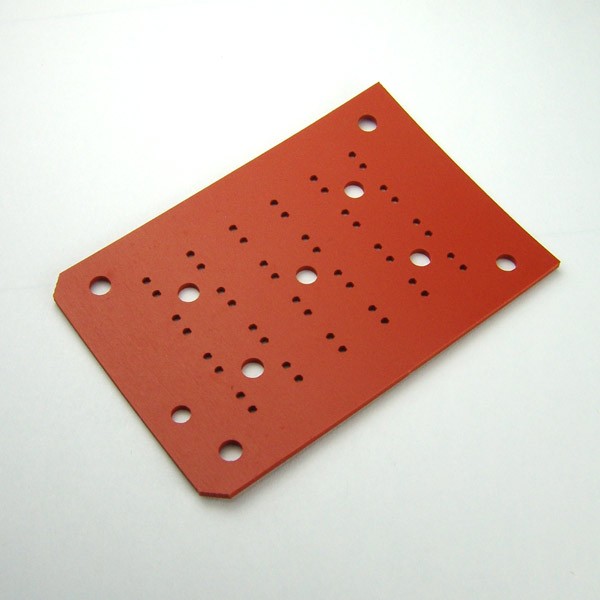
HTe‾Chem Electrochemistry
 Analytical is pleased to offer a new setup for High-Throughput Experimentation for Electrochemistry (HTe‾Chem). This new line of products allows broad electrochemical reactions (such as electrosynthesis, organic electrochemistry, electrophotochemistry, etc.) to be carried out on Analytical Sales standard 24-well minirack platform. Simply speaking, it turns Analytical Sales’ minirack into a high-throughput microscale electrochemical reactor.
Analytical is pleased to offer a new setup for High-Throughput Experimentation for Electrochemistry (HTe‾Chem). This new line of products allows broad electrochemical reactions (such as electrosynthesis, organic electrochemistry, electrophotochemistry, etc.) to be carried out on Analytical Sales standard 24-well minirack platform. Simply speaking, it turns Analytical Sales’ minirack into a high-throughput microscale electrochemical reactor.
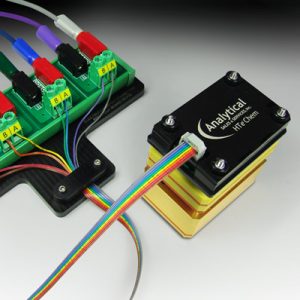
Analytical Sales HTe‾Chem platform allows you to accelerate your electrochemistry workflow by permitting multiple simultaneous constant current, constant voltage, and/or electrophotochemical experiments to be run.
Choose between 2 setups; one optimized for constant current, and one optimized for constant voltage.
The constant current setup allows the user to set a precise current, which will be maintained by the power supply throughout the reaction. The voltage will automatically be varied based on the changing electrical conditions as the reaction progresses.
The constant voltage setup allows the user to set a precise voltage, which will be maintained by the power supply throughout the reaction. In this setup, the current will automatically be varied based on the changing electrical conditions as the reaction progresses.
Analytical Sales offers a myriad of electrode choices which can be used as either cathodes or anodes depending on your experimental needs. If your goal is to determine which electrode(s) work best, we offer an electrode kit.
Analytical Sales HTe‾Chem platform is fully compatible with our 24-LED, 9mm-spacing Lumidox platform, allowing experimentation with electrophotochemical reactions.
More information on HTe‾Chem can be found here:
Article: Electrochemical Recycling of Adenosine Triphosphate in Biocatalytic Reaction Cascades
Video: https://pubs.acs.org/doi/suppl/10.1021/acscentsci.1c00328/suppl_file/oc1c00328_si_004.mp4

HTe‾Chem Assemblies & Kits
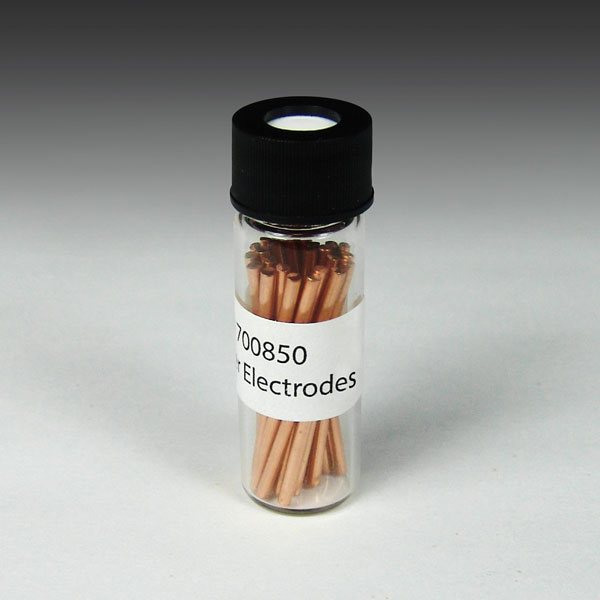
Electrodes for HTe‾Chem
Analytical Sales offers a myriad of electrode choices for use in HTe‾Chem Assemblies. They can be used as either cathodes or anodes depending on your experimental needs. If...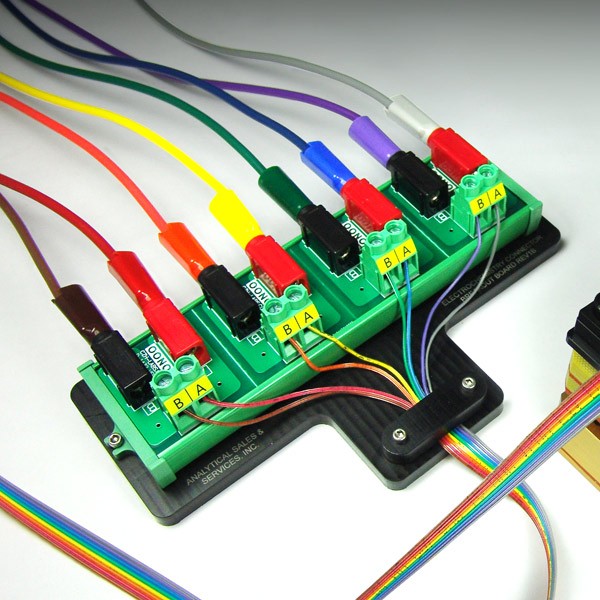
Power Supply & Accessories